The Importance of pH:
The issue of ocean Acidification linked to Climate Change now has a a serious implication for Shellfish Producers. Their website reflects these concerns: http://bcsga.ca/ocean-acidification/
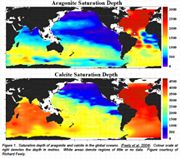 A few physical factors have a disproportionate effect on the distribution of organisms and the fact that humans play a large role in their modification means that their effects on the sustainability of ecosystems is rather importantaragonite, ph
A few physical factors have a disproportionate effect on the distribution of organisms and the fact that humans play a large role in their modification means that their effects on the sustainability of ecosystems is rather importantaragonite, ph
Canadian Science Advisory Secretariat Research Document – 2008/013 State of physical, biological, and selected fishery resources of Pacific Canadian marine ecosystems(Page 37 of pdf file) Ocean acidification off the West Coast by Debby Ianson, Fisheries and Oceans Canada “Global oceans are becoming more acidic due to increasing carbon dioxide (Orr et al. 2005). Much of the extra CO2 released by burning fossil fuels ends up in the oceans, increasing the dissolved inorganic carbon concentration (DIC). As DIC increases, the relative proportions of carbon species shift (specifically from the carbonate ion to the bicarbonate ion), resulting in an increase in acidity and a decrease in pH (Strum and Morgan, 1981).
At present the pH of seawater has decreased by about 0.1 due to oceanic uptake of anthropogenic carbon and is projected to decrease by 0.4 by the year 2050 (Orr et al. 2005). The decrease in pH (and concurrent decrease in carbonate ion) means that organisms that produce calcite and aragonite shells or structures, such as pteropods, corals and shellfish, are threatened (The Royal Society, 2005).” “Very few data from the carbonate system have been collected on the Canadian west coast; however these few observations show that Juan de Fuca Strait and the Vancouver Island Coastal Current experience high pCO2 water due to tidal mixing in the Strait, which brings water high in DIC and low in pH to the surface (Ianson et al. 2003). An additional study with high spatial resolution confirms the high surface pCO2 (400 — 800 ppm; Nemcek et al, in press) in this area estimated by Ianson et al. (2003) but has no complimentary measurements (such as DIC) with which to determine pH in the Strait.”
From “WATER: http://www.unep.org/geo/geo4/report/04_Water.pdf Rainwater and ocean acidification Acidity in rainwater is caused by the dissolution of atmospheric CO2, as well as by atmospheric transport and deposition of nitrogen and sulphur compounds (see Chapters 2 and 3). This is important because biological productivity is closely linked to acidity (see Chapter 3). The box on acidifying cycles in Chapter 3 describes some of the impacts of acid deposition on the world’s forests and lakes. The oceans have absorbed about half of the global CO2 emissions to the atmosphere over the past 200years (see Chapter 2), resulting in the increasing acidification of ocean waters (The Royal Society 2005). Acidification will continue, regardless of any immediate reduction in emissions. Additional acidification would take place if proposals to release industrially produced and compressed CO2 at or above the deep sea floor are put into practice (IPCC 2005). To date, injection of CO2 into seawater has been investigated only in small-scale laboratory experiments and models. Although the effects of increasing CO2 concentration on marine organisms would have ecosystem consequences, no controlledecosystem experiments have been performed in the deep ocean nor any environmental thresholds identified. The impacts of ocean acidification are speculative, but could be profound, constraining or even preventing the growth of marine animals such as corals and plankton. They could affect global food security via changes in ocean food webs, and, at the local scale, negatively affect the potential of coral reefs for dive tourism and for protecting coastlines against extreme wave events. It is presently unclear how species and ecosystems will adapt to sustained, elevated CO2 levels (IPCC 2005). Projections give reductions in average global surface ocean pH (acidity) values of between 0.14and 0.35units over the 21st century, adding to the present decrease of 0.1 units since pre-industrial times(IPCC 2007). Managing water issues related to climate change Global-scale changes to the water environment associated with climate change include higher sea surface temperatures, disruption of global ocean currents, changes in regional and local precipitation patterns, and ocean acidification. These issues are typically addressed through global efforts, such as the UN Framework Convention on Climate Change and its Kyoto Protocol (see Chapter 2). Management at the global level involves numerous actions at regional, national and local scales. Many global conventions and treaties are implemented on this basis, with their effectiveness depending on the willingness of individual countries to contribute to their achievement. Because these changes are linked to other environmental issues (for example, land use and biodiversity), they must also be addressed by other binding or non-binding treaties and instruments (see Chapter 8). Major responses to the drivers of climate change – primarily the increased burning of fossil fuels for energy – are analysed in Chapter 2. These responses are generally at the international level, and require concerted action by governments over the long-term, involving legal and market- driven approaches. Focus is on responses to climate change-related impacts affecting the water environment that involve regulation, adaptation and restoration
Pacific Ocean acid levels jeopardizing marine life
Vancouver Island researchers use artificial tide pools to study threat
From CBC News
Posted: Jul 17, 2012 2:17 AM PT
Last Updated: Jul 17, 2012 12:19 PM PT
Very few data from the carbonate system have been collected on the Canadian west coast; however these few observations show that Juan de Fuca Strait and the Vancouver
Island Coastal Current experience high pCO2 water due to tidal mixing in
the Strait, which brings water high in DIC and low in pH to the surface
(Ianson et al. 2003).
An additional study with high spatial resolution
confirms the high surface pCO2 (400 — 800 ppm; Nemcek et al, in press) in
this area estimated by Ianson et al. (2003) but has no complimentary
measurements (such as DIC) with which to determine pH in the Strait.”
The foillowing is taken from the publication:”WATER”
“http://www.unep.org/geo/geo4/report/04_Water.pdf”
Rainwater and ocean acidification : Acidity in rainwater is caused by the dissolution
of atmospheric CO2, as well as by atmospheric transport and deposition of
nitrogen and sulphur compounds (see Chapters 2 and 3). This is important
because biological productivity is closely linked to acidity (see Chapter
3). The box on acidifying cycles in Chapter 3 describes some of the
impacts of acid deposition on the world’s forests and lakes. The oceans
have absorbed about half of the global CO2 emissions to the atmosphere
over the past 200years (see Chapter 2), resulting in the increasing
acidification of ocean waters (The Royal Society 2005). Acidification will
continue, regardless of any immediate reduction in emissions. Additional
acidification would take place if proposals to release industrially
produced and compressed CO2 at or above the deep sea floor are put into
practice (IPCC 2005). To date, injection of CO2 into seawater has been
investigated only in small-scale laboratory experiments and models.
Although the effects of increasing CO2 concentration on marine organisms
would have ecosystem consequences, no controlled ecosystem experiments have
been performed in the deep ocean nor any environmental thresholds
identified. The impacts of ocean acidification are speculative, but could
be profound, constraining or even preventing the growth of marine animals
such as corals and plankton. They could affect global food security via
changes in ocean food webs, and, at the local scale, negatively affect the
potential of coral reefs for dive tourism and for protecting coastlines
against extreme wave events. It is presently unclear how species and
ecosystems will adapt to sustained, elevated CO2 levels (IPCC 2005).
Projections give reductions in average global surface ocean pH (acidity)
values of between 0.14and 0.35units over the 21st century, adding to the
present decrease of 0.1 units since pre-industrial times(IPCC 2007).
Managing water issues related to climate change Global-scale changes to the water
environment associated with climate change include higher sea surface
temperatures, disruption of global ocean currents, changes in regional and
local precipitation patterns, and ocean acidification. These issues are
typically addressed through global efforts, such as the UN Framework
Convention on Climate Change and its Kyoto Protocol (see Chapter 2).
Management at the global level involves numerous actions at regional,
national and local scales. Many global conventions and treaties are
implemented on this basis, with their effectiveness depending on the
willingness of individual countries to contribute to their achievement.
Because these changes are linked to other environmental issues (for
example, land use and biodiversity), they must also be addressed by other
binding or non-binding treaties and instruments (see Chapter 8). Major
responses to the drivers of climate change – primarily the increased
burning of fossil fuels for energy – are analyzed in Chapter 2. These
responses are generally at the international level, and require concerted
action by governments over the long-term, involving legal and market-
driven approaches. Focus is on responses to climate change-related impacts
affecting the water environment that involve regulation, adaptation and
restoration .



